Science
What are defect phases and defect phase diagrams?
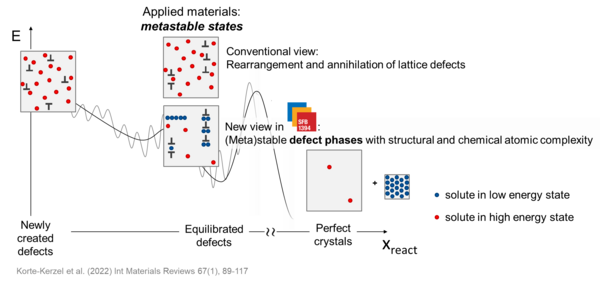
We aim to provide a framework to consider two essential design parameters for material scientists together: the stability of different crystalline phases and the lattice defects contained in them. This will enable us to develop a powerful materials design tool.
We do this by taking the structural and chemical atomic complexity known to exist at defects as our starting point.
Structural and chemical atomic complexity Complex arrangements of atoms forming a defect in terms of atomic positions and elemental species, as opposed to the surrounding lattice with crystallographic order based on simpler structural units with random or regular chemical occupation. |
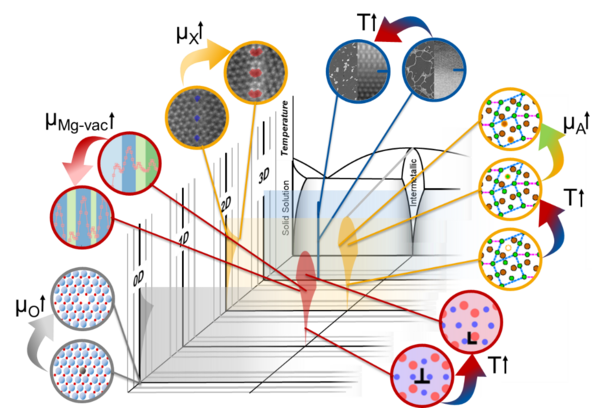
By structural and chemical atomic complexity, we understand complex arrangements of all atoms forming a defect in terms of their positions and elemental species, as opposed to the surrounding lattice with crystallographic order based on simpler structural units with random or regular chemical occupation. Examples from our first funding phase of defects in Mg-Al-Ca with such distinct atomic structures and how these can change are shown below.
Defect phase Structurally and chemically distinct atomic-scale defect configuration which exists over a region of thermodynamic state variables such as temperature, stress, alloy composition, chemical potential, etc. → Defect Phase Diagram |
As a result of considering the formation of specific structural and chemical order at defects, we can explore their stability with (meta)stable configurations referred to as defect phases. We define a defect phase as a structurally and chemically distinct atomic-scale defect configuration which remains unchanged over a region of thermodynamic state variables such as temperature, stress, alloy composition, chemical potential, etc. Such defect phases can undergo phase transitions, which will impact their properties. By analysing prevalent defect phases for different values of thermodynamic state variables one arrives at the defect phase diagram.
For defect phase diagrams, the main variable, equivalent to temperature and bulk composition in conventional bulk phase diagrams, is the chemical potential. It is constant across a defect phase, its matrix and surroundings. The chemical potential therefore presents a coordinate system for all defect phase diagrams.
More concrete examples of defect phase diagrams from our research and how these are linked to material properties can be found here on three topics:
Grain boundaries in Mg alloys
Dislocations in Laves phases
Planar defects in Mg-Al-Ca solid solutions