Projects of SFB1394
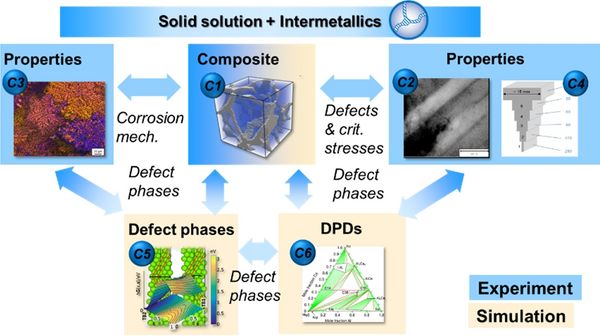
The central research questions posed in this SFB are concerned with the atomic structure and chemistry as well as the thermodynamics of defect phases governing material properties. The defect phases yield a natural structure based on their dimensionality. We have therefore organised our individual projects in three project areas defined by the dimensionality of the principal defect phases considered:
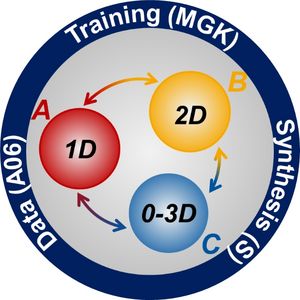
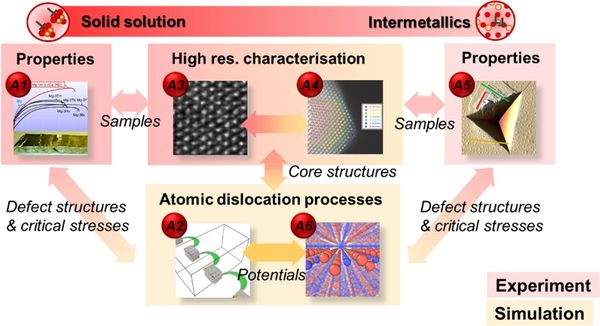
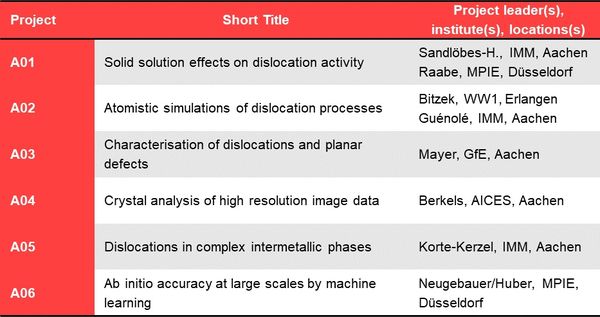
Project Area A: 1D defect phases - For more detail on each project, please choose the corresponding link on the left.
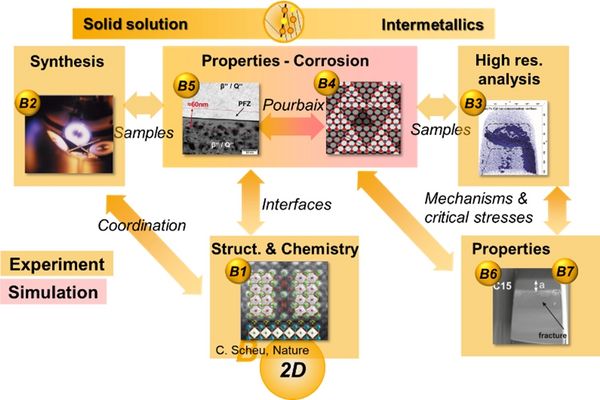
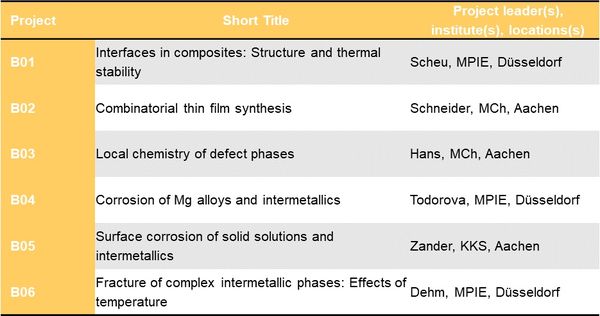
Project Area B: 2D defect phases - For more detail on each project, please choose the corresponding link on the left.
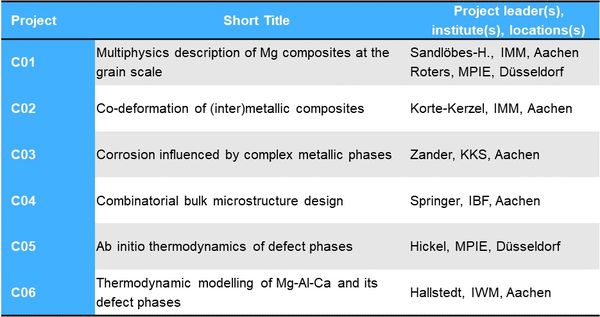
Project Area C: 3D composites and 0D-3D concepts - For more detail on each project, please choose the corresponding link on the left.